Polyethylene (PE) is one of the most widely used and versatile plastic materials globally, prized for its cost-effectiveness, lightweight properties and ease of formability. These characteristics make PE indispensable across a broad spectrum of applications, from packaging materials to structural plastics.
However, despite its widespread utility, PE’s inherent chemical inertness limits its functionality in advanced applications, holding back its potential for more innovative uses.
To unlock this potential, it’s essential to introduce polar functional groups into PE, which can significantly enhance its properties and open doors to new applications. This challenge has become a major focus in polymer chemistry, where developing efficient methods to modify PE has drawn increasing interest.
One of the primary obstacles in modifying PE is its chemical resistance, which makes it difficult to functionalize using conventional methods. This inertness also contributes to the buildup of PE waste in landfills, posing a severe environmental challenge. As plastic pollution continues to threaten ecosystems worldwide, finding ways to recycle or upcycle PE into valuable products is imperative.
Although some approaches exist for modifying PE, they often lack scalability or efficiency, underscoring the need for innovative solutions that balance environmental impact with functional benefits for this ubiquitous plastic.
Among the various strategies available, amination has emerged as one of the most promising methods for modifying PE.
But why focus on amines?
Amines are nitrogen-based groups with one or more hydrogen atoms attached in the form of N-H bonds. These N-H groups can engage in hydrogen bonding, enabling interactions between polymer chains. This not only enhances the polymer’s chemical reactivity but also improves its performance across a variety of applications, from adhesives to coatings.
However, achieving efficient functionalization of PE with amines has been a significant challenge; most methods require multiple, energy-intensive steps or risk degrading the polymer’s properties.
Consequently, progress toward a scalable, effective method for aminating PE has been limited. Notably, converting the N-H groups in aminated PE into other species could further broaden PE’s range of applications.
This is where my research, as a postdoctoral fellow in the Schafer group at the University of British Columbia, makes a bold leap forward.
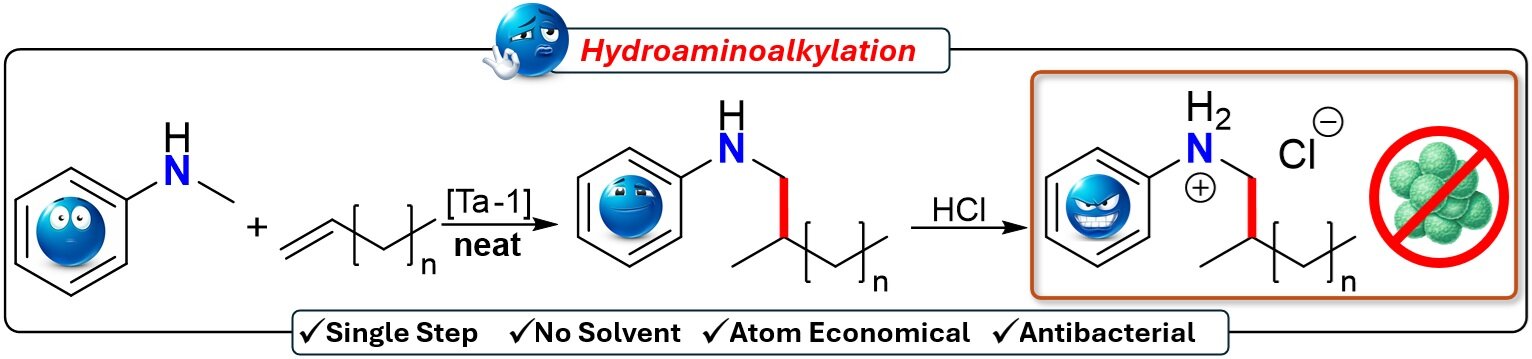
Through extensive exploration, I focused on a catalytic process called hydroaminoalkylation to aminate PE efficiently. Previously used in modifying polypropylene, this technique showed promise in transforming PE through a simple, one-step reaction.
The work is published in the journal Angewandte Chemie International Edition.
The strength of this method lies in its efficiency: It works under mild, solvent-free conditions and avoids the radical-induced degradation seen in conventional methods. By applying this approach to vinyl-terminated polyethylene (VTPE), generously provided by my industrial partner NOVA Chemicals, I successfully created amine-functionalized PE with minimal reaction steps, enhancing scalability and cost-effectiveness.
At the University of British Columbia, my colleagues in the Hatzikiriakos group conducted rheological and mechanical tests on my aminated PE. They found that introducing amine groups not only modified the chemical properties of PE but also impacted its physical characteristics.
For instance, the crystallization temperature of the modified PE increased, indicating stronger intermolecular interactions within the polymer due to the amine groups. The amine groups also increased the material’s hydrophilicity (or attraction to water), as the N-H bonds can form hydrogen bonds with water molecules.
Traditionally, recycling PE has been challenging due to its chemical inertness, but this new approach allows us to upcycle PE waste into valuable resources. Aminating waste PE holds significant promise for sustainability, making it possible to reuse and repurpose it for various applications.
Now, imagine a world where you don’t need to sanitize your hands every time you touch a surface, a common concern during the COVID-19 pandemic. This is the problem I aimed to address in my study.
Here’s how amination can lead to an antibacterial effect: I converted the amine groups in the polymer into positively charged ammonium groups by treating the aminated PE with a hydrochloride solution. Since bacteria have negatively charged cell membranes, they are naturally attracted to the positively charged ammonium groups.
This electrostatic interaction disrupts the bacterial cell membrane, ultimately killing the bacteria. After this transformation, my collaborators at the University of Calgary, the Heyne team, exposed the modified polymer to Staphylococcus aureus bacteria. The polymer killed all the bacteria in a short exposure time.
In essence, I developed an antibacterial polymer that holds potential for use as a coating on everyday surfaces, offering a way to prevent the spread of germs without the need for constant sanitizing.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about Science X Dialog and how to participate.
More information:
Saeed Ataie et al, Hydroaminoalkylation for Amine Functionalization of Vinyl‐Terminated Polyethylene Enables Direct Access to Responsive Functional Materials, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202410154
I am a postdoctoral fellow at the University of British Columbia in the Schafer group. My research is focusing on designing catalysts for hydroaminoalkylation of polyolefins. Prior to my postdoctoral, I had completed my Ph.D. in inorganic chemistry and catalysis at the University of Ottawa, in the Baker group.
Citation:
Transforming polyethylene: From functionalization to antibacterial properties for sustainable applications (2024, November 9)
retrieved 9 November 2024
from j91
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.