Cereals such as rice, wheat, maize, and barley are essential in the human diet and have various uses in the food industry. Their suitability for different industrial applications depends on the properties of their grains. The major component of these cereal grains is starch, which is a glucose polymer found exclusively in plants.
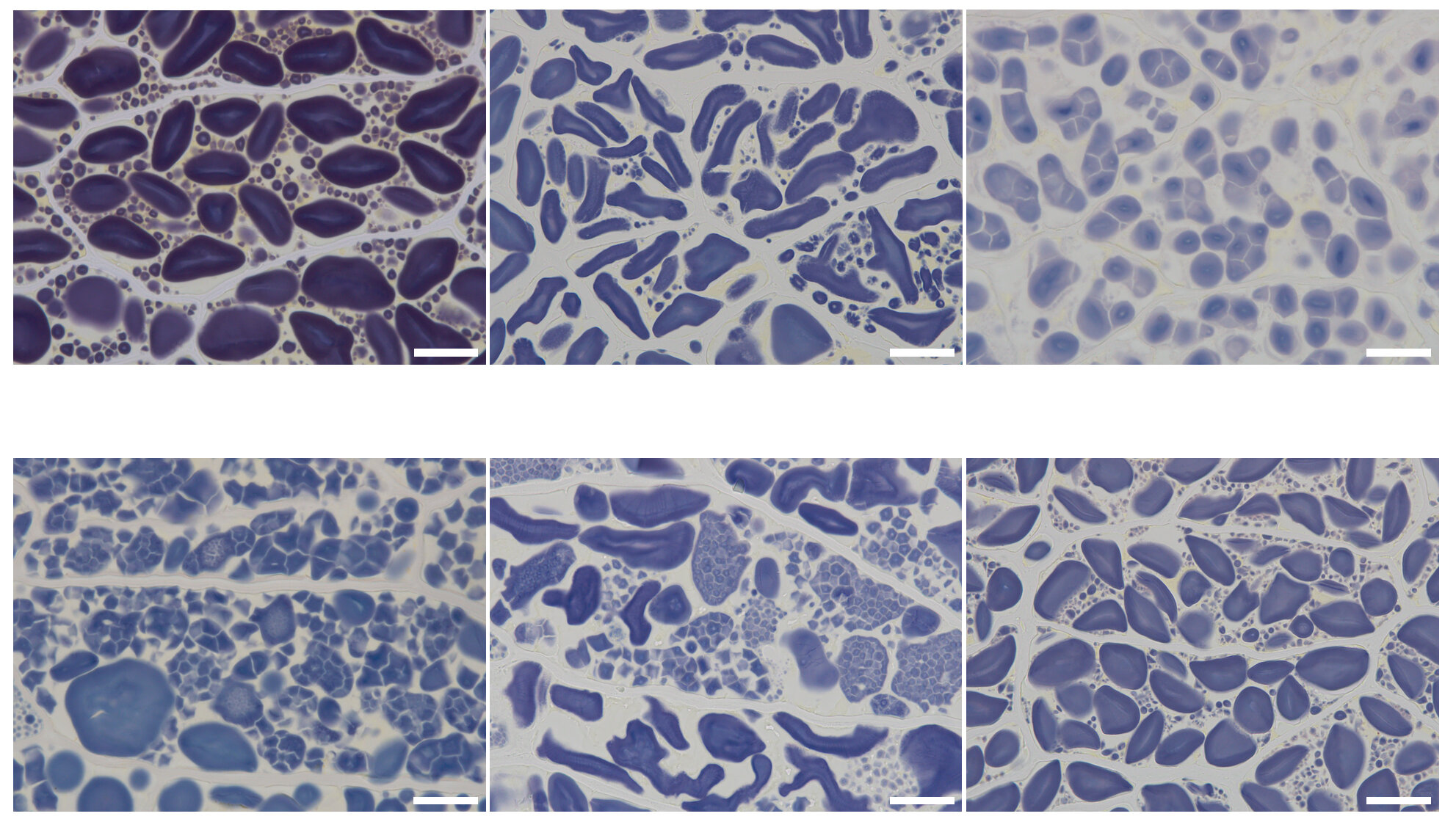
Starch forms particles known as starch granules (SGs) within plant cells. SGs are classified as either simple or compound. A simple SG consists of a single starch particle, while a compound SG is made up of multiple starch particles. The types of SGs vary depending on the plant species. For example, barley, wheat, and maize endosperms contain simple SGs, while rice endosperm develops compound SGs.
Specific enzymes involved in the synthesis of amylopectin, a major component of starch, dictates the shape and size of SGs. The key steps involved in amylopectin synthesis are elongation of glucose chains by starch synthases (SSs), branching of these chains by branching enzymes (BEs), and removal of misplaced glucose branches by starch debranching enzymes (DBEs).
When some of these enzymes are absent or dysfunctional due to a genetic variation called mutation, it leads to considerable changes in the starch properties. For instance, when a DBE is absent, like in ISOAMYLASE1 (ISA1) mutants of barley (hvisa1), the malformed glucose branches prevent the formation of amylopectin and leads to the accumulation of phytoglycogen, that has an extensive branching with short glucan chains and has a molecular weight significantly lower than that of amylopectin. Similar characteristics were observed in sweet corn varieties of maize.
Although the genetic interactions among mutations in starch biosynthesis genes are well-studied in rice and maize, not much is known in barley. To bridge this gap, Associate Professor Ryo Matsushima from the Institute of Plant Science and Resources, Okayama University explored the genetic interactions among different starch biosynthesis genes in barley, and published their study in Theoretical and Applied Genetics on 31 August, 2024.
Dr. Matsushima says, “In this study, based on our screening for SG morphology, we isolated starch BE mutants in barley, hvbe2a-1, and hvbe2a-2, which develop elongated SGs in endosperm, unlike in the wild-type barley.” Analyzing these mutants, they found that hvbe2a-1 had a base change in HvBE2a gene, leading to an amino acid substitution which disrupts the enzyme activity, while in hvbe2a-2, HvBE2a was absent due to a chromosomal deletion.
Previously, Dr. Matsushima’s group had reported reduced starch content with an increase in phytoglycogen in a DBE mutant barley, hvisa1, and this trait was enhanced by mutations on the hvflo6 gene. They had also reported that in hvisa1 mutants, compound SGs were formed in the endosperm, in contrast to the simple SGs in the wild-type barley. When they crossed a hvisa1 mutant with the hvbe2a mutants, they found that the hvisa1 hvbe2a double mutants formed simple SGs like in the wild-type.
“This suggests that the absence of HvBE2a, a major BE in barley endosperm, is expected to reduce the formation of incorrect glucose chains, decreasing the need for trimming by HvISA1. As a result, the loss of HvBE2a has the potential to alleviate the observable trait caused by hvisa1 mutation,” comments Dr. Matsushima.
Some mutations affecting SG morphology in endosperms also affect the SG morphology in cereal pollen. They found that the suppressive effect of hvbe2a against hvisa1 mutation was not observable in pollen, which contrasts with their findings in the endosperm. These results indicate different pathways for maintaining normal SGs in pollen and endosperm. The exact pathways and genetic interactions driving the structural changes of SGs in cereals is an evolving field of research.
Dr. Matsushima says, “Our findings provide new insights into genetic interactions in the starch biosynthetic pathway, demonstrating how specific genetic alterations and combination of mutations can influence starch properties and SG morphology, with potential applications in cereal breeding for desired starch properties.”
More information:
Ryo Matsushima et al, Mutations in starch BRANCHING ENZYME 2a suppress the traits caused by the loss of ISOAMYLASE1 in barley, Theoretical and Applied Genetics (2024). DOI: 10.1007/s00122-024-04725-7
Provided by
Okayama University
Citation:
Understanding the influence of specific gene mutations on starch properties in barley (2024, October 23)
retrieved 24 October 2024
from VD5
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.